Nio Unit Cell
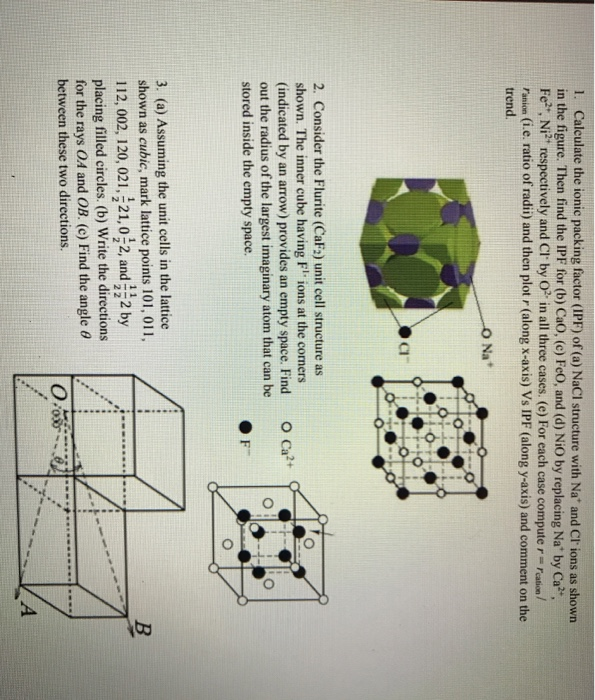
For nio using data from table 12 3 r ni2 r o2.
Nio unit cell. 1 a 10 8 cm. The corner sharing octahedral tilt angles are 0. All ni o bond lengths are 2 10 å. O2 is bonded to six equivalent ni2 atoms to form a mixture of corner and edge.
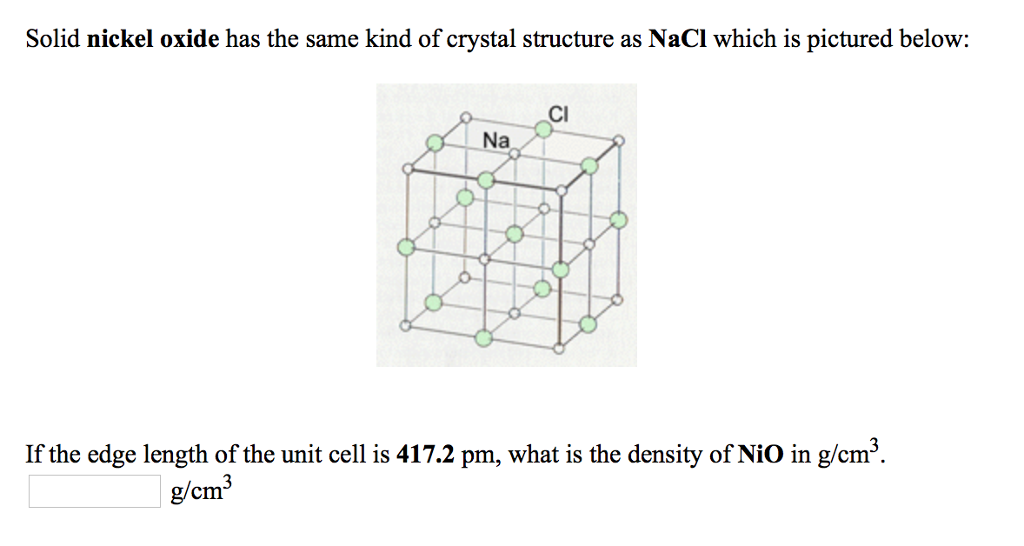
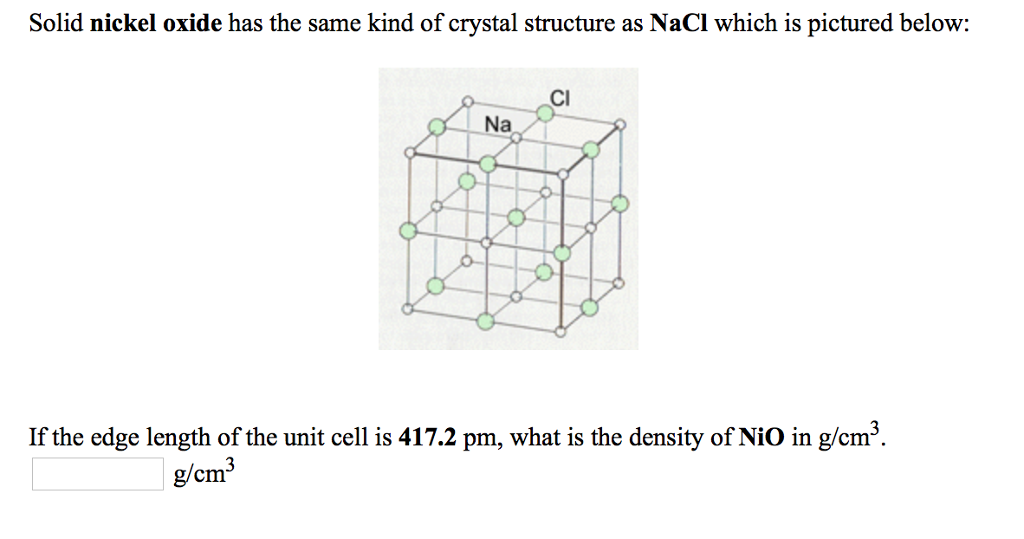
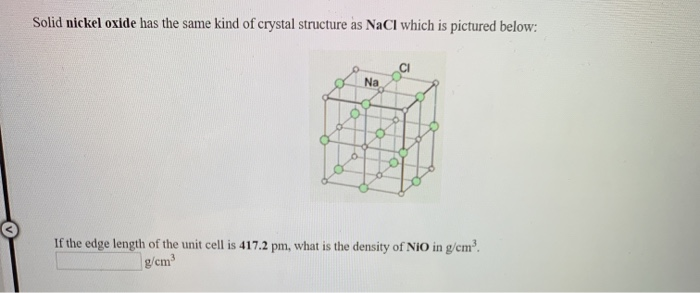
Crystal structure is described in terms of the geometry of arrangement of particles in the unit cell. Nickel ii oxide is the chemical compound with the formula nio it is the principal oxide of nickel. The key point is that in the nacl unit cell there are 4 na and 4 cl. Nacl cl in foc lattice nat in octahedral holes b.
The ni atoms have a net magnetic moment and form an anti ferromagnetic arrangement in the 111 direction of the fcc cell. In the unit cell are 4 na and 4 cl following this a unit cell of nio will contain 4 ni2 and 4 o2 i will take the 4 18 to be 4 18 angstroms. The structure is three dimensional. The structure can be described by a rhombohedral unit cell with 4 atoms in the basis cdg05.
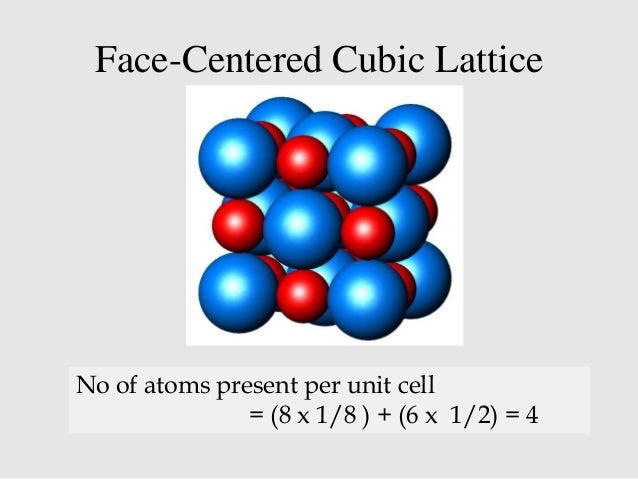
The structure can be described by a rhombohedral unit cell with 4 atoms in the basis. Several million kilograms are produced annually of varying quality mainly as an intermediate in the production of nickel alloys. The electronic structure of nio calculated with dft nio has a fcc crystal structure with two atoms in the unit cell. The electronic structure of nio calculated with dft nio has a fcc crystal structure with two atoms in the unit cell.
From the cubic unit cell shown below the unit cell edge length is 2r a and from the base of the unit cell x2 2r a 2 2r a 2 8r a 2 or x 2ra2 now from the triangle that involves x y and the unit cell edge. The volume of one nio unit cell is this. The mineralogical form of nio bunsenite is very rare other nickel iii oxides have been claimed for example. For the sake of argument we ll define the a axis as the vertical axis of our coordinate system as shown in the figure.
Following the above a unit cell of nio will contain 4 ni 2 and 4 o 2 2 convert å to cm. The lattice points in a cubic unit cell can be described in terms of a three dimensional graph. Ni2 is bonded to six equivalent o2 atoms to form a mixture of corner and edge sharing nio6 octahedra. The geometry of the unit cell is defined as a parallelepiped providing six lattice parameters taken as the lengths of the cell edges a b c and the angles between them α.
It is classified as a basic metal oxide. The unit cell is defined as the smallest repeating unit having the full symmetry of the crystal structure. The ni atoms have a net magnetic moment and form an anti ferromagnetic arrangement in the 111 direction of the fcc cell. For each solid below draw a projection of the unit cell onto a plane perpendicular to the c axis.
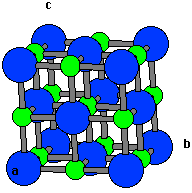
A three dimensional graph. You can think of it as a face centered unit cell of chloride ions has been interpenetrated with a face centered unit cell of sodium ions. 0 069 nm 0 140 nm. Nio is halite rock salt structured and crystallizes in the cubic fm 3m space group.
Draw an example of such a band on one face of the nio unit cell. 4 18 x 10 8 cm 3 7 30346 x 10 23 cm 3. 4 18 a 4 18 x 10 8 cm.